So what happens in your body when you take cannabis or cannabinoids?
This primarily depends on which cannabinoids were taken and how they were taken/the route of administration.
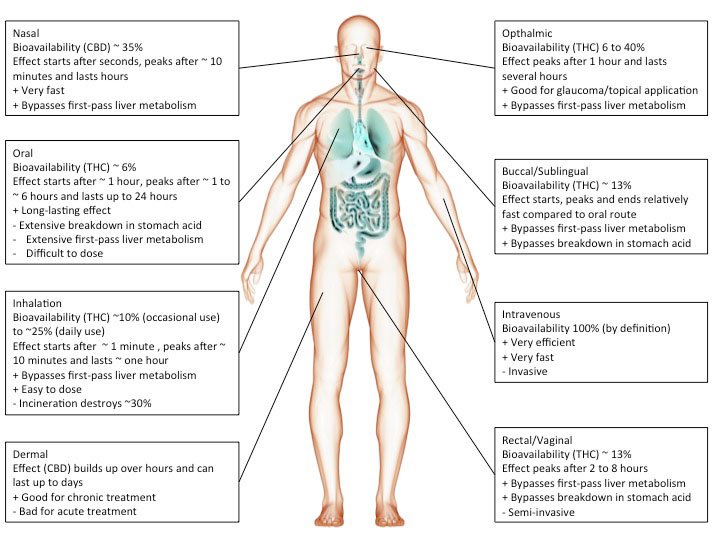
Route of administration
Cannabis or cannabinoids are often smoked but can also be ingested (oil, cake, tea etc.), injected or absorbed through the skin (patch) or mucous membranes (tongue, cheek, rectum). The chosen route of administration has a major impact on the actual effect.
For instance, smoking inevitably heats up cannabinoids causing decarboxylation and a shift from acidic to neutral compounds, which tend to be more biologically active. In addition, the incineration process will render part of the cannabinoids biologically inactive.
However, inhaled cannabinoids enter the bloodstream through the lungs and therefore reach the rest of the body before it passes the liver. This first-pass liver effect can seriously reduce the amount of available cannabinoids. Similarly, ingesting cannabinoids can reduce the amount of available cannabinoids through degradation in the gut/digestive tract.
Heat, chemical degradation or biological degradation/metabolism can dramatically change the amount of cannabinoids that is biologically active: bioavailability. Injected substances are considered 100% bioavailable. Any reduction is ascribed to the route of administration.
Smoked, or inhaled cannabinoids have reported bioavailabilities ranging from 2-56%(Huestis, 2007) with an average of about 30% (McGilveray, 2005). This variability is mainly due to differences in smoking dynamics (how deeply does one inhale, how long does one hold it in). Compared to oral application, the effects of smoking cannabinoids are relatively fast in on- and offset.
cannabinoids that are absorbed through the mucous membranes in the mouth (buccomucosal application) have bioavailabilities of around 13% (Karschner et al., 2011). Application via the mucous membranes of tongue and cheek or rectal application bypasses degradation in the gut and improves bioavailability.
When cannabinoids are ingested, bioavailability is reduced to about 6% (Karschner et al., 2011).
Development
CB1 can be detected in human tissues throughout development (Mato et al., 2003). Comparison of CB1 distribution in the human brain at difference developmental stages shows:
-CB1 expression is relatively low in fetuses, intermediate in children and high in adults.
-CB1 expression in adults is particularly high in hippocampus and basal ganglia.
-CB1 expression in fetuses is particularly high in capsula interna, pyramidal tract and brachium conjunctivum (while not detectable in children and adults) suggesting a transient surge of CB1 in developing neuronal fiber tracts and thus a role in brain development.
Example by numbers
Now let’s breakdown what happens when you smoke a cannabis cigarette (adapted from (McGilveray, 2005)).
A cigarette containing 35 mg THC produces peak THC levels of 162 ng/ml plasma 10 minutes after onset of smoking.
An average human has approximately 5l of blood meaning that 162 x 5000 = 810 μg of THC is bioavailable at peak levels, corresponding to bioavailability of 0.81/35 = 2.4% at peak levels.
Peak THC levels are 162 ng/ml. The molecular weight of THC is 314g/mol. 314 ng of THC in 1 ml of plasma corresponds to a 1 μM THC concentration. Therefore, our measured concentration of 162 ng/ml corresponds to 520 nM THC at peak. After 30 minutes, ~20% or ~100 nM is still bioavailable. After 2 hours, ~3% or 15 nM of THC is still bioavailable.
With relative affinities for THC of 10 nM and 24 nM for CB1 and CB2 receptors it can be assumed that most biological activity of THC will have disappeared within 2 hours after smoking a cannabis cigarette (An affinity of 10 nM means that at this concentration 50% of receptors are bound/activated by THC).
Literature
Huestis, M.A. (2007). Human cannabinoid Pharmacokinetics. Chem. Biodivers. 4, 1770–1804.
Karschner, E.L., Darwin, W.D., Goodwin, R.S., Wright, S., and Huestis, M.A. (2011). Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin. Chem. 57, 66–75.
Mato, S., Del Olmo, E., and Pazos, A. (2003). Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur. J. Neurosci. 17, 1747–1754.
McGilveray, I.J. (2005). Pharmacokinetics of cannabinoids. pain Res. Manag. J. Can. pain Soc. J. Société Can. Pour Trait. Douleur 10 Suppl A, 15A – 22A.